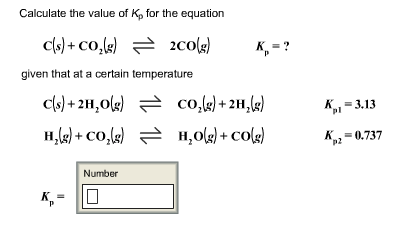
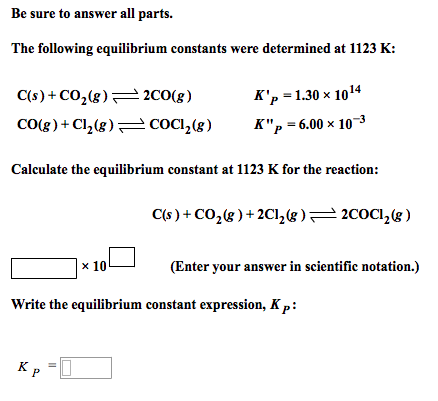
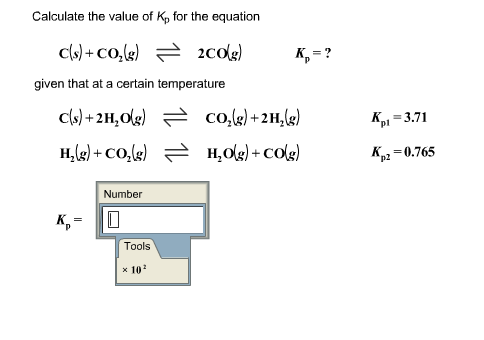
3.For the equilibrium C(s)+ COg) gives 2CO(g) Kp is 63 atm at 1000 K. If at equilibrium Pco=10Pco2 then total pressure at equilibrium is : 4.A(g) is 90

Solve this: â ‹Q3 For the equilibrium C(s) + CO2(g) ⇌2CO(g) KP = 63 atm at 1000 K - Chemistry - Chemistry in Everyday Life - 11997217 | Meritnation.com

For the reaction: C (s)+ CO2 (g) 2CO (g) the partial pressure of CO2 and CO are 2 and 4 atm respectively at equilibrium. Then equilibrium costant for the reaction is -
The value of K for the reaction Co2(g) + C(s) (eqi. symbol) 2Co is 3.0 at 1000K If initially pCO=0.48 bar and pCO= 0 bar and pure graphite is present. calculate the

Values of the kinetic constant of the reaction C+CO2→2CO as a function... | Download Scientific Diagram

CH4 and CO2 conversions in the reaction of DRM (CH4 + CO2 → 2H2 + 2CO)... | Download Scientific Diagram