GönüL DostLarı - D VİTAMİNİ EKSİKLİĞİ NEDENİYLE ORTAYA ÇIKTIĞI PEK BİLİNMEYEN 7 HASTALIK‼️ Alerjik rahatsızlıklardan kansere kadar birçok hastalığa neden olan D vitamini eksikliğini dünyada birçok insan yaşıyor... 👉D Vitamini Eksikliği Hastalıkları:

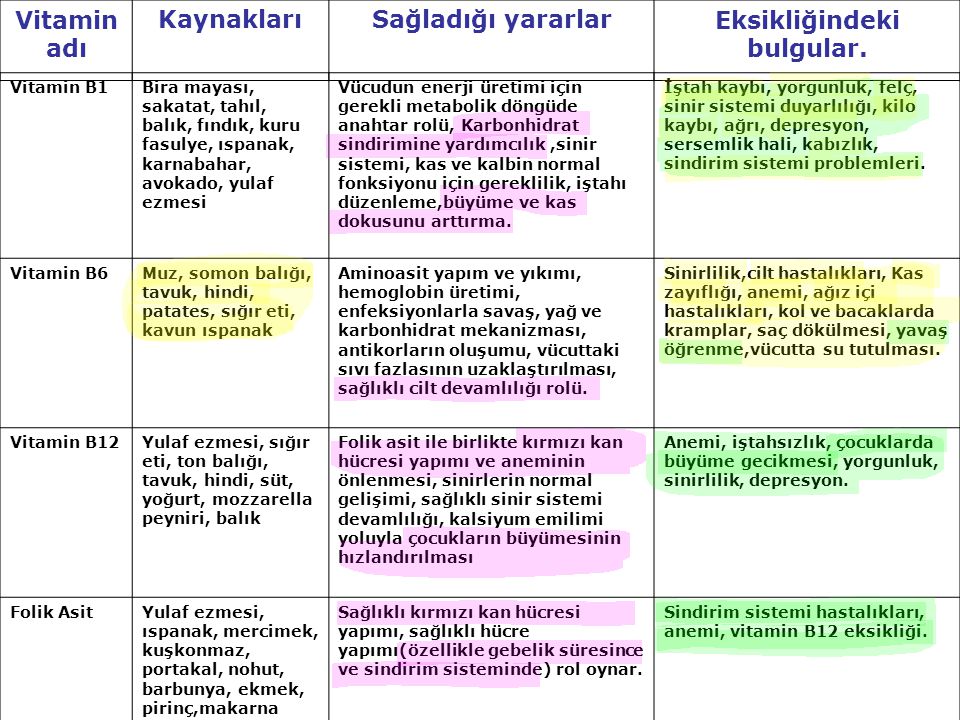
ya vitaminler ve vitamin eksikliğinde görülen hastalıkları tablo halinde bulamıyorum yardımcı olun lütfe.. :( - Eodev.com