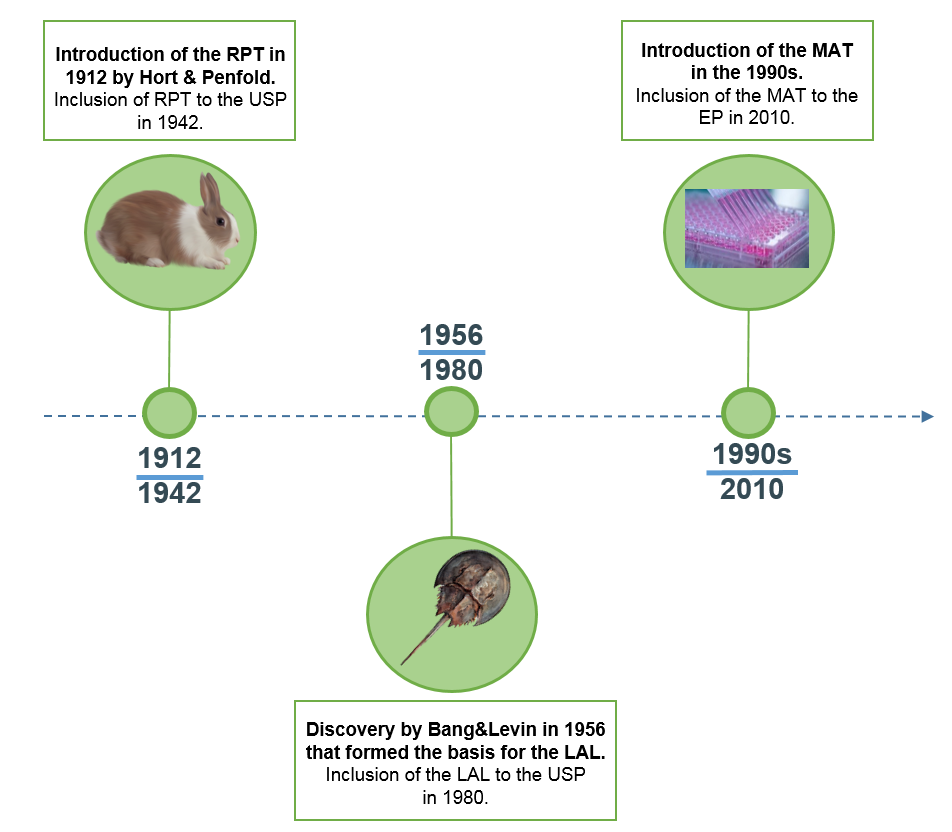
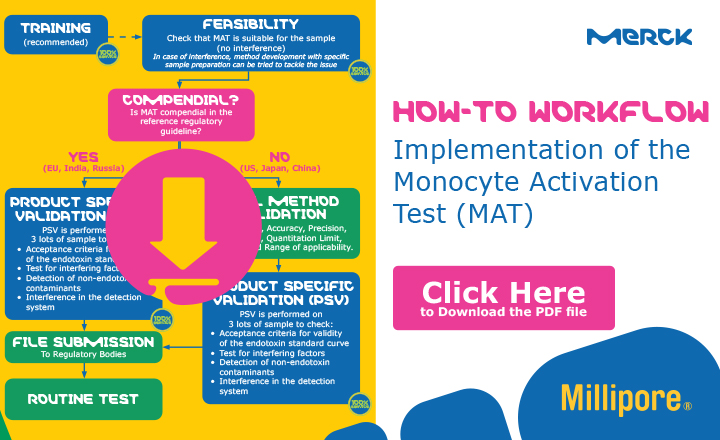
Pyrogen Testing Revisited on Occasion of the 25th Anniversary of the Whole Blood Monocyte Activation Test. - Document - Gale OneFile: Health and Medicine

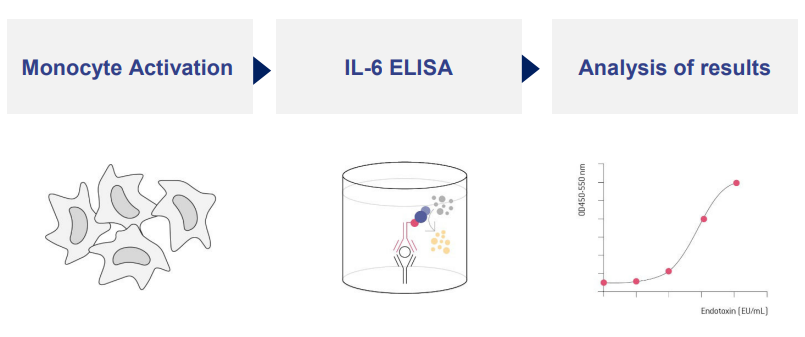
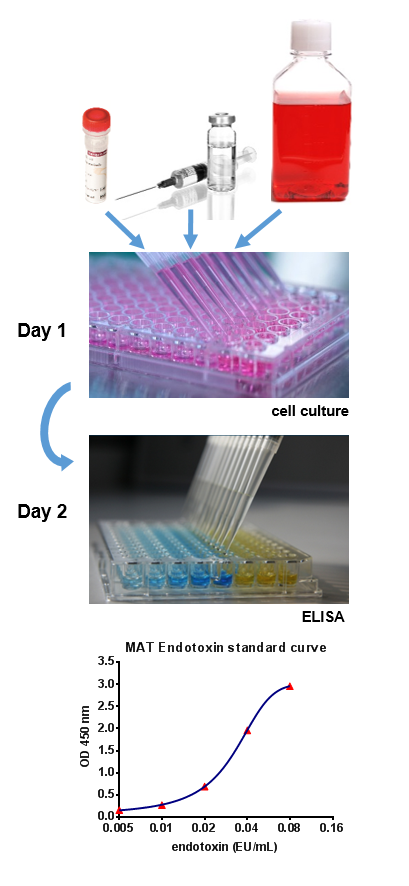
The biological principle of the "Monocyte Activation Test" as defined... | Download Scientific Diagram
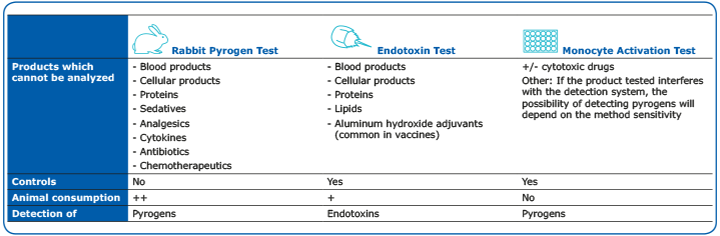
Pyrogen Testing Revisited on Occasion of the 25th Anniversary of the Whole Blood Monocyte Activation Test. - Document - Gale OneFile: Health and Medicine