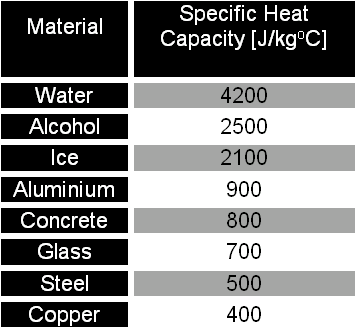
The amount of heat energy required to convert 1 kg of ice at - 10^∘C to water at 100^∘C is 7,77,000 J. Calculate the specific latent heat of ice. Specific heat capacity

Calculate the heat required to convert 3 kg of ice at - 12^o C kept in a calorimeter to steam at 100^o at atmospheric pressure. (Given: specific heat of ice = 2.100 ×
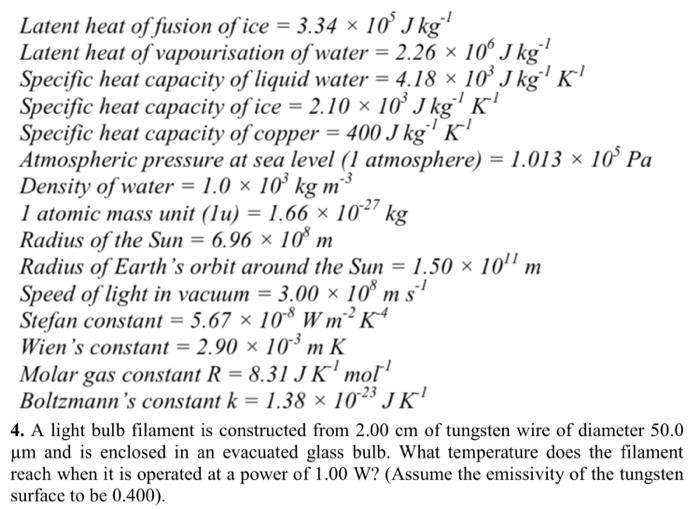
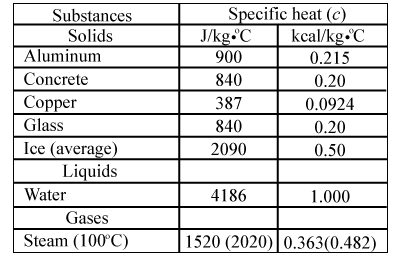
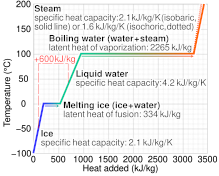
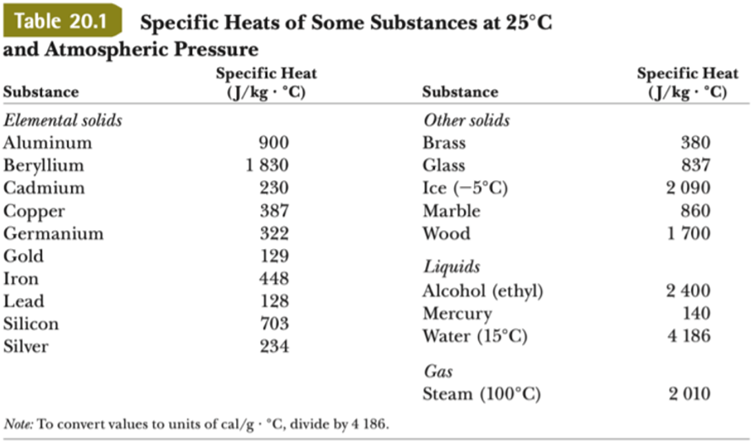
What are the values of the specific heat capacity of water, steam, and ice (in both joule and cal.) and the values of latent heat of fusion and vaporisation for water? -
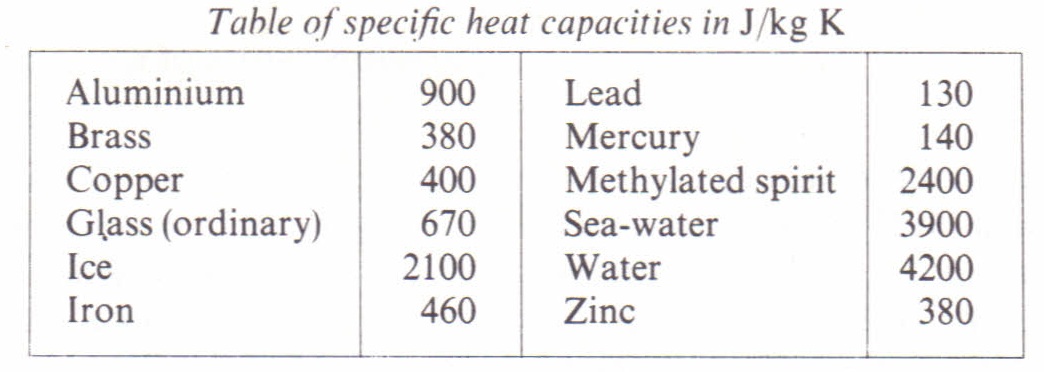
Specific heat capacity Physics Homework Help, Physics Assignments and Projects Help, Assignments Tutors online

Calculate the heat required to convert 3 kg of ice at - 12^o C kept in a calorimeter to steam at 100^o at atmospheric pressure. (Given: specific heat of ice = 2.100 ×

The temperature of 1.94 kg of water is 34 °C. To cool the water, ice at 0°C is added to it. The desired - brainly.com

Calculate the heat required to convert 3 kg of ice at `-12^()C` kept in a calorimeter to steam a... - YouTube
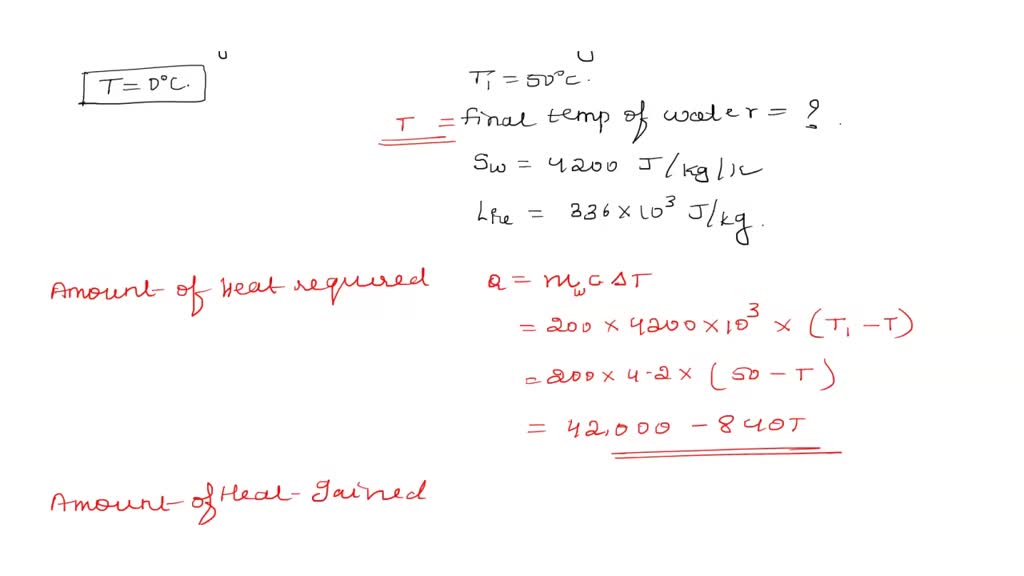
SOLVED: A piece of ice of mass 40 g is added to 200 g of water at 50oC. Calculate the final temperature of water when all the ice has melted. Specific heat
What is the difference between specific latent heat of melting of ice and specific latent heat of fusion of ice? - Quora